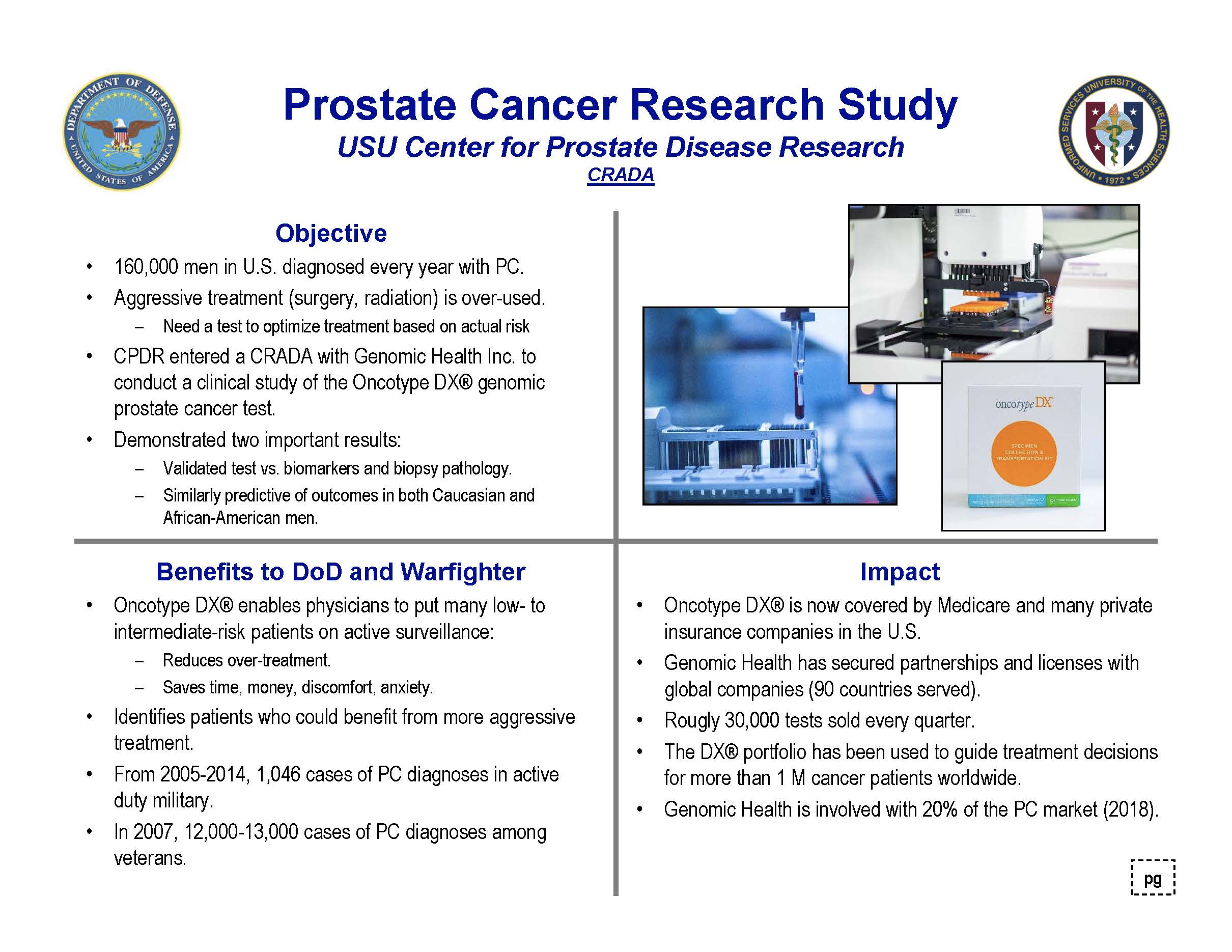
Genomic Health Inc. (GHI) Oncotype DX® products detect the biology of a patient’s tumor to optimize cancer treatment decisions. The overall goal of the HJF-USU-GHI collaborative study was to evaluate the ability of the biopsy-based molecular assay to provide critical information regarding the likelihood of having aggressive disease for individual patients with early-stage prostate cancer at the time of diagnosis.
Oncotype DX® enables physicians to put many low- to intermediate-risk patients on active surveillance and therefore reduces over-treatment of these patients, and physicians identify patients who could benefit from more aggressive treatment. Overall, Oncotype DX® was shown guide treatment decisions that reduce over-treatment, and save time, money, discomfort, and anxiety in patients. Additionally, study results demonstrated similarly predictive outcomes in both Caucasian and African-American men.
Oncotype DX® is now covered by Medicare and many private insurance companies in the U.S., and the DX® portfolio has been used to guide treatment decisions for more than 1 million cancer patients worldwide.
Genomic Health has secured partnerships and licenses with global companies in 90 countries served, and in 2018, Genomic Health reported it has captured about 20 percent of the prostate cancer market.