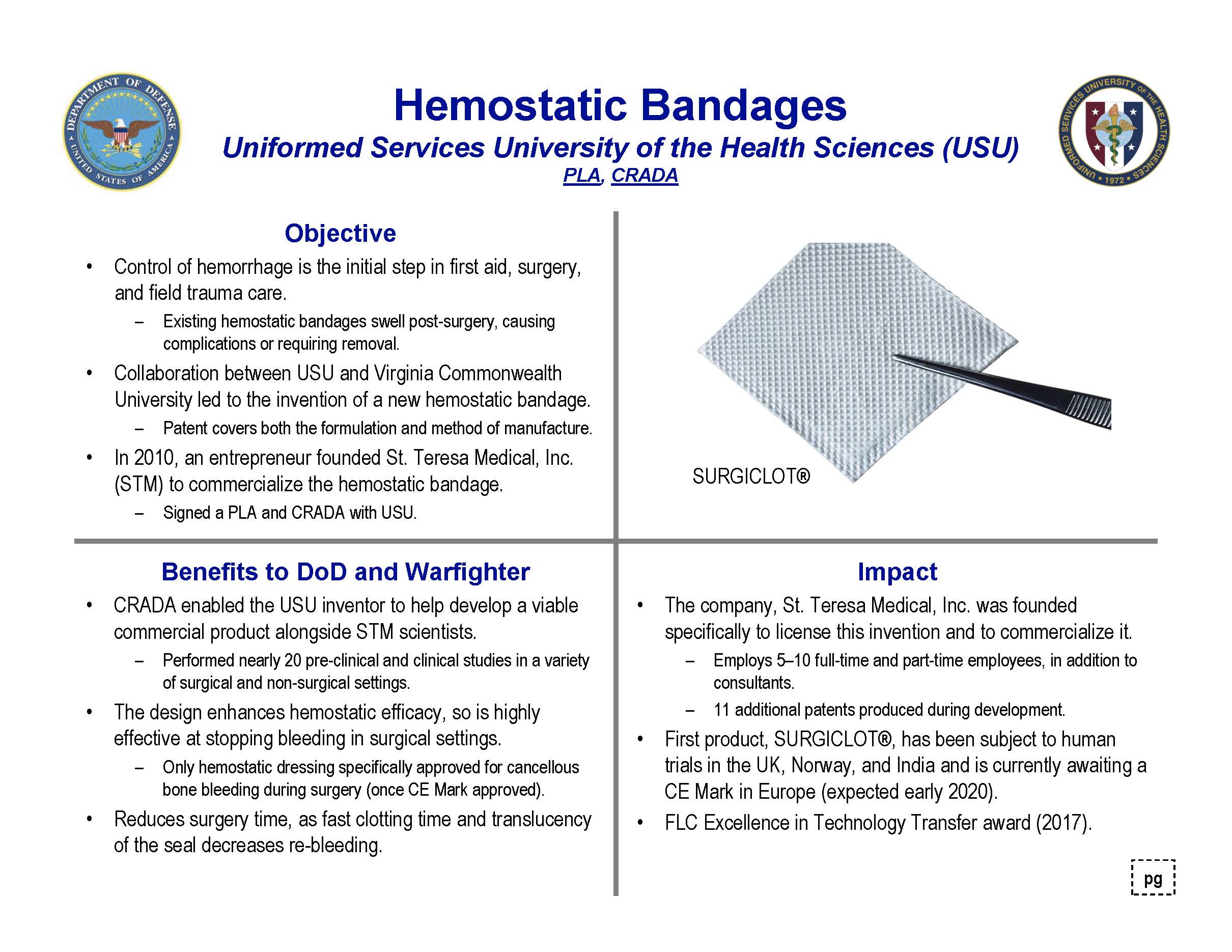
A collaboration between Dr. Stephen Rothwell at the Uniformed Services University (USU) and Dr. David Simpson at Virginia Commonwealth University (VCU) led to the invention of a hemostatic bandage, and this USU–VCU invention was patented. In 2010 an entrepreneur interested in the hemostatic bandage invention founded St. Teresa Medical, Inc., a start-up medical device manufacturing company based in Minnesota, with the intent to commercialize the invention.
The bandage invention was exclusively licensed by HJF and VCU to St. Teresa Medical. A number of cooperative research and development agreements (CRADAs) between HJF, USU and St. Teresa Medical (STM) enabled the USU inventor to help develop and evaluate a viable commercial product, SURGICLOT®, a dissolvable dressing that leaves behind the essential human clotting proteins, fibrinogen and thrombin, at the injury site. This is the only hemostatic dressing specifically for cancellous bone bleeding during surgery (and has a CE mark approval pending).
The first product, SURGICLOT®, has been subject to human trials in the UK, Norway and India and is currently awaiting a CE Mark in Europe, with nearly 20 pre-clinical and clinical studies performed in a variety of surgical and non-surgical settings, where the design enhanced hemostatic efficacy. It was highly effective in reducing surgery time, as fast clotting time and translucency of the seal decreased re-bleeding. The bandage also received a Federal Laboratory Consortium for Technology Transfer (FLC) Excellence in Technology Transfer award in 2017.